Abstract
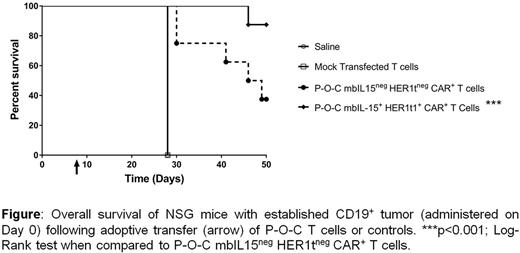
Chimeric antigen receptor (CAR)-modified autologous T cells have shown remarkable clinical responses targeting B-cell malignancies. However, a majority of manufacturing approaches rely on expensive viral vectors and ex vivo activation of clinical-grade T cells for gene transfer with subsequent 2-3 week-long numeric expansion to achieve necessary dosing. We have engineered our clinically-validated Sleeping Beauty (SB) system for stable non-viral integration of CAR, cytokine signaling, and safety switch transgenes into T cells to eliminate need for viral vectors. We previously demonstrated that co-expression of membrane bound IL-15 (mbIL15, Proc Natl Acad Sci U S A. 2016 Nov 29;113(48):E7788-E7797) on CD19-specific CAR+ T cells using SB system allows for very rapid (<2 days) manufacturing with point-of-care (P-O-C) technology (Blood 2016 128:2807). This approach to production avoids the need for activation and propagation of CAR+ T cells leading to augmented persistence and anti-tumor activity in an in vivo model of leukemia. We now update this technology by co-expressing a safety switch, HER1t, on CD19-specific CAR+ mbIL15+ T cells. HER1t improves the safety profile by providing a mechanism for selective in vivo depletion of P-O-C-produced T cells through the administration of cetuximab, a clinically-available monoclonal antibody. We confirmed co-expression of CAR, mbIL15, and HER1t in P-O-C-manufactured T cells and demonstrated that these cells exhibited a memory-like phenotype, potent antigen-specific cytotoxicity, and produced IFNγ, TNFα, and IL-2 when co-cultured with CD19+ tumor cells. Cetuximab specifically and efficiently killed mbIL15+ HER1t+ CAR+ T cells via antibody dependent cellular cytotoxicity and complement dependent cytotoxicity in a dose-dependent manner. Adoptive transfer of mbIL15+HER1t+CAR+ T cell generated in less than 2 days under P-O-C into immunocompromised (NSG) mice bearing established CD19+ leukemia (NALM-6) resulted in significant reduction in tumor burden and improvement in overall survival, compared to control mice (Figure). Blood analysis demonstrated improved expansion and persistence of mbIL15+ HER1t+ CAR+ T cells compared to mbIL15negHER1tnegCAR+ T-cell control. In summary, we have generated T cells co-expressing mbIL15 and the HER1t safety switch in less than two days using P-O-C that demonstrated superior efficacy and persistence compared to P-O-C T cells that expressed CAR without mbIL15. These pre-clinical data demonstrate the effectiveness of very rapidly manufactured mbIL15+ HER1t+ CAR+ T cells in targeting CD19+ tumors and provide a rationale for clinical evaluation.
Chan: Intrexon Corporation: Employment. Gallagher: Intrexon Corporation: Employment. Cheng: Intrexon Corporation: Employment. Carvajal-Borda: Intrexon Corporation: Employment. Plummer: Intrexon Corporation: Employment. Govekung: Intrexon Corporation: Employment. Barrett: ZIOPHARM Oncology: Employment. Khare: Ziopharm Oncology: Employment. Cooper: Immatics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Miltenyi Biotec: Honoraria; Ferring: Consultancy; Intrexon Corporation: Equity Ownership, Patents & Royalties; Argos Therapeutics: Equity Ownership; Targazyme, Inc.: Equity Ownership; Ziopharm Oncology: Employment, Equity Ownership, Research Funding; Organovo Holdings: Equity Ownership; Procter & Gamble: Equity Ownership; AmpliPhi Biosciences: Equity Ownership; Sangamo Biosciences: Patents & Royalties. Shah: Intrexon Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal